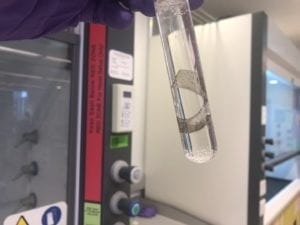
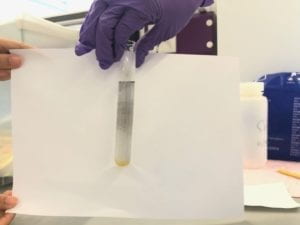
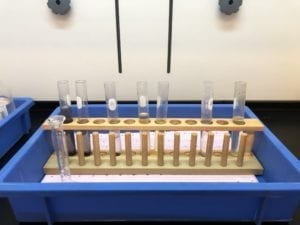
3.2 EAPs Manufacturing
- Cut Nafion-117 into 2 x 1cm x 5cm, 2 x 2cm x 5cm, 2x 1cm x 10cm, 1 x 2cm x 10cm.
- Roughen the surface of Nafion-117 polymer by sandpapering.
- Prepare an aqueous solution of the complex ([Pt(NH3)4]Cl2 or [Pt(NH3)6]Cl4).
- Place the one cut of the polymer in the stirring Pt solution at 60◦C for 1 hour.
- When Pt particles have immersed into the polymer, add 5% N2H4·H2SO4 solution.
- When grey metallic layers form on the membrane surface, finish the experiment.
- Repeat the process.
3.3 Performance Testing
Dry Test:
Using ATTEN TPR3003T’s Regular DC Power Supply and 2 pieces of conductive tape on both sides of the IPMCs polymer.
Modify the voltage from 0V to 30V to activate the polymer. Then modify the current from 0.1A to 10A to supply different intensity electric field level with the combination of various voltage.
Underwater Test:
Using ATTEN TPR3003T’s Regular DC Power Supply and 2 pieces of wire with an insulating layer on the side surface touching both sides of the IPMCs polymer.
Modify the voltage from 0V to 30V to activate the polymer. Then modify the current from 0.1A to 3A to supply different intensity electric field level with the combination of various voltage.
Experiment Results Analysis
First Experiment Result: Failed
Reasons’ Analytic Report:
- The previous stirring time is not enough for Pt ions to attach on the surface of the polymer.
- The NaBH4 solution was mixed with the Pt solution, which caused waste of the Pt ions in solution.
- The concentration of the ammonium hydroxide solution is too high and caused the high pH level of the solution. Finally, it results in the precipitation of Pt.
Second Experiment Result: Half Success
Reasons’ Analytic Report:
- The oxygen in the open air oxidized the Pt that was successfully attached to the polymer.
- The Pt on the polymer is still not enough after a single round of the experiment. The above procedures need to be repeated 3-5 times to ensure the efficiency of the polymer.
Third Experiment Result: Half Success
Reasons’ Analytic Report:
- Tried to use a higher concentration of NaBH4 solution. However, it turned out to be too much. The Pt that had already attached on the surface in previous steps drop down because of the fierce bubbles caused by the reaction.
- The time is so limited that the reaction is not fully completed.
Fourth Experiment Result: Success
Reasons’ Analytic Report:
- Cut the edge of the polymer in order to avoid the electricity to go through the edge to another surface.
- Substituted the NaBH4 by the N2H4·H2SO4. This change narrowed down the cost of manufacture.
Figures and Videos for Step 3.2 & 3.3