Introduction
Our idea come from two perspectives:
1. After the locomotion lab, we realized that although we tried to make robotic arms and legs, we cannot avoid the use of motors, servos and other metallic compartments. Although they seem to function well, they are different from real animal movements. In real world, no animal have this type of rotating body part. Thus, they have certain limitations and drawbacks. As the real animals’ locomotion is driven by muscles, we want to develop real artificial muscles for new foundation of robotic locomotion.
2. We at first want to make a jellyfish floating in the air. Then after research we discovered jellyfish move by propulsion using the muscle in its body.

The muscle expand to create a negative pressure to inhale water, then contract to propel them out. So we shift our focus on developing an artificial muscle.
After research, we focused on a new material: Ionic Polymer-Metal Composites (IPMCs), which is a subset of Electroactive Polymers (EAPs). This kind of material can conduct movements such as shrink and stretch under a low voltage. It can function as artificial muscles for a new foundation of robotics design. Then we researched its property and mechanism, and designed experiment to manufacture it.
EAPs and IPMCs
We researched on the development and mechanism of IPMCs and EAPs:
EAPs is firstly invented by Wilhelm Rontgen. Then in the 1960s, a major breakthrough of EAPs took place, and the first electrically conducting polymers were discovered by Hideki Shirakawa. In the early 1990s, IPMCs were developed and appears to be much more effective compared to EAPs. It needs only 1 or 2 volts to motivate, and this became a significant discovery for the bio-inspired robotics’ development.
We discovered that EAP materials have two categories, Dielectric Electroactive Polymers (DEAP), whose squeezing and stretching behavior is caused by electrostatic forces on the surfaces. The force stretches and squeezes the insulated polymers in the middle. This type of material is resilient and relatively easy to manufacture. However, it requires thousands of volts to function. The other type is Ionic Electroactive Polymers (IEAP), which is represented by IPMCs. The material is made up of an cation or anion exchange polymer with its two sides plated by metals. When being added voltages, the surfaces creates an electric field in the polymer, moving the corresponding ion to one side. However, the detailed theory behind its behavior is not fully understood.
However, the artificial muscle made of IPMCs is still hard to be manufactured, and the quality of the material is still beyond control. Thus, base on previous research done by other scientists, we want to find a simple procedure for common students to produce their own artificial muscle from EAPs, and find ways to apply this new kind of artificial muscle to the bio-inspired robotics.
Manufacturing Procedure
we studied various materials including PDMS, Nafion, Platinum, Carbon. After thourough research, we discovered Nafion 117 and Platinum is a great material combination for making IPMCs.
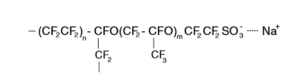
The structure of Nafion 117
We decided to use NaBH4 to reduce Pt ion to nanometer scale Pt metal particles after we let Pt ion sink into the polymer, based on the formula:
NaBH4 + 4Pt2+ +8OH− ⇒ 4Pt + 16NH3 + NaBO2 + 6H2O.
Below are the manufacturing guide proposed by us based on research and experiment:
Material Preparation
Equipment: Beaker, Glass Rod, Dropper, Test Tube, Forceps, Water Bath, pH test paper.
Reagents: 10cm x 10 cm Nafion-117 Polymer, Platinum standard solution, diluted hydrochloric acid, 1% ammonium hydroxide solution, 10% NaBH4
Manufacturing
1. Cut Nafion-117 into 2 x 1cm x 5cm, 2 x 2cm x 5cm, 2x 1cm x 10cm, 1 x 2cm x 10cm.
2. Roughen the surface of Nafion-117 polymer by sandpapering.
3. Mix Pt solution with ammonium hydroxide until pH is 3.
4. Place the one cut of the polymer in the stirring Pt solution at 80◦C for 5 minutes.
5. Move the polymer to NaBH4 solution at 50◦C, observed bubbles emerge on the polymer. Wait for the reaction to be over.
6. Repeat process 4-5
7. When dense grey metallic layers form on the membrane surface, finish the experiment.

Our lab
Lab Record
First Experiment:
To test what is the best pH for Pt solution, we first made the pH = 6, yet a unexpected reaction happened and precipitate most Pt ion.

high pH caused Pt ion, chlorine ion and ammnonium ion to form ammonium chloroplatinate
Then we followed the instruction of a paper to mix Pt solution with NaBH4, which cause more trouble, as the Pt ion in the solution react directly with NaBH4.
The result of mixing Pt solution with NaBH4 together.
First Experiment Result: Failed
Reasons’ Analytic Report:
The previous stirring time is not enough for Pt ions to attach on the surface of the polymer.
The NaBH4 solution was mixed with the Pt solution, which caused waste of the Pt icons in solution.
The concentration of the ammonium hydroxide solution is too high and caused the high pH level of the solution. Finally, it results in the precipitation of Pt.
From it we designed new procedure proposed in the beginning.
Second Experiment:
We follow our procedure clearly and successfully plated Platinum on the polymer. However, we didn’t repeat 4,5 process enough and the nanometer scale Pt scatter loosely on the polymer, which are quickly oxidized after we took it out.

Polymer in Reaction: The bubbles are ammonium.

Polymer plated with nanoscale Pt
Second Experiment Result: Half Success
Reasons’ Analytic Report:
The Pt that was successfully attached to the polymer was oxidized by the oxygen in the open air.
The Pt on the polymer is still not enough after a single round of the experiment. The above procedures need to be repeated 3-5 times to ensure the efficiency of the polymer.
We knew we need to repeat procedure 4 and 5 until a dense layer is formed on the whole surface of Nafion.
Third Experiment
In the last repetition of step 5, we tried reduction agent with higher concentration to accelerate. However the reaction is too violent that the bubbles flushed all Pt particles down to the bottom of the test tube.

Too violent reaction
Third Experiment Result: Half Success
Reasons’ Analytic Report:
Try to use a higher concentration of NaBH4 solution, however it turned out to be too much and the Pt that had already attached on the surface in previous steps, drop down.
The time is so limited that the reaction is not fully completed.
Fourth Experiment: Succeed!
We used hydrazine sulfate as reduction agent this time and we applied it in a very diluted solution with a very long reaction time (almost 12 hours).

The contraction is very slight because it is a air test. The material performs better in solution base. Yet it is easier to take photos and observe it in air.
…
This material can act as a new foundation for robot locomotion system design. We have studied several feasible applications:
1 Heart, Lung, and Jellyfish:
Heart, lung and the propulsion locomotion of jellyfish are similar: the muscle expands, creating a negative pressure to draw in blood, air or water, then the muscle contracts to push them out. They can be implemented by our material.
Using the IPMCs based artificial muscle as the jellyfish’s muscle underwater. The Microbit and related editor like mu-editor can be used to do the physical computing part and provide periodical voltage. The basic movement like moving forward and blood cycle can be achieved by applying different intensity of electric field.
2 Arms, Legs, and Bio-Inspired Seahorse Robotic:
The Seahorse Robotic might be the best robotic to fully utilize the feature of IPMCs based artificial muscle. By using the IPMCs with less Pt density to build the top half part of the seahorse robotic and use the more Pt density one to build the bottom half. The IPMCs’ feature of having different shrinking and stretching level under different Pt density and electric field intensity. It is similar with robotic arms and legs, where the IPMCs connects with to bones, which are assembled by a joint. (From our paper)
Paper link:
https://docs.google.com/document/d/14qt9JRaHoY7bswd0Ce1aVMzVlq7tvD74nGl1PTJUozk/edit?usp=sharing
NYU account login need to open the link and track our progress.