Electrons orbits of a Helium atom.
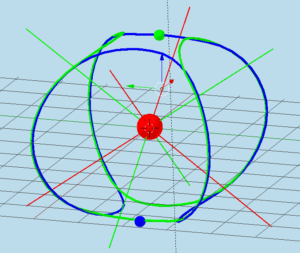
Figure 1. The shape of electrons orbits of a Helium atom in the para- configuration, which corresponds to the ground state of an atom. The orbits of two electrons are shown with different colors (first electron – blue, second electron – green). The straight lines originated from the nucleus show the directions of orbital moments and the directions of induced magnetic fields for each electron.
Abstract.
Our analysis of the electron orbit for a Helium atom repeats several aspects of our analysis of the electron orbit of a Hydrogen atom, because these are the same types of orbits. Taking into account that the Hydrogen atom has only one electron, our solution was not strictly the only possible solution for the electron orbit.
In the case of the Helium atom, there is only one solution for two electrons, which create both dipole and quadrupole moments. Additional restrictions can be used for model control, because ortho- and para- configuration of electron orbits have their specific sets of energy levels.
We present here a simple solution as well as a detailed image of an electrons orbits in Helium atoms. Both para- and ortho- configurations of electron orbits are analyzed. We explain why the ground state of a Helium atom is not the lowest energy state.
Quantum Mechanical expressions for Hamiltonians for both Helium and Hydrogen do not include the term for Maxwell Electrodynamics. Magnetic fields induced by rotating electrons are simply ignored.
We combine Electrodynamics and Quantum Mechanics in order to calculate the exact parameters of orbits.
The Pauli Principle postulates electrons spin directions as up and down. This Principle needs to be postulated in Quantum Mechanics, because it contradicts both the Law of Energy Conservation and also Electrostatics. We demonstrate that actual directions of spins are radial directions toward the center of the nucleus and away from the center of the nucleus. Our model explains Pauli Principle, but does not need a postulate.
Orbital moments of electrons in our model align along the radii of the electrons orbits. They can have directions toward and away from the center of the nucleus. In our model, electron spins are aligned along the magnetic fields created by the orbital movement of electrons. Electron spins behave similar to a compass, which aligns along the stronger magnetic field.
Complicated energy spectra of a Helium atom gets a simple explanation in the terms of two types of orbits and two sets of energy levels for the ortho- and para- Helium.
We use Quantum Mechanics the same way as N. Bohr used for his model of the Hydrogen atom, but we do not use operators, so we are not bound by the statistical characteristics of the Uncertainty Principle.
In the same approach as we used for the Hydrogen atom orbit we do not need to use the Quantum Mechanical Orbital Postulate, Pauli Postulate or any other postulates.
Introduction & the Current state of the problem.
Quantum Mechanical orbitals indicate that the maximum probability density to find an electron in an atom is located inside the proton in a Hydrogen atom. Electron orbit is calculated as the convolution of the shape of the orbital and the experimentally suggested shape of spherical shells.
For a Helium atom, that approach does not work. That is why besides the circular shape of the electron orbit there is no calculation of the actual shape of the electrons orbit in a Helium atom.
Experiments prove that in the case of a Helium atom the difference between ortho-Helium and para-Helium is not restricted to having opposite spin. It is different atomic orbit configurations with different sets of energy levels. The nature of that difference is not discussed.
In Project 2, we shall address these problems and discuss other questions.
In the previous part, we indicated that for a Hydrogen atom the differential approach can produce several types of solutions. The presence of only one electron made it quite difficult to choose a correct solution for a single dipole moment. Two electrons in a Helium atom create both a dipole and quadrupole moment, as well as restricting parameters of every part of orbit to the single quarter of a sphere. Combined with the noble behavior in chemical reactions, these conditions give us the opportunity to find a single solution.
Electron spins directions.
First we have to make a note about the directions of spins and about the term of spin-orbital interaction.
- Individual spins of electrons in a Helium atom are equal to one half. The total spin of a Helium atom in the ground state is equal to zero. From a Mathematics point of view, this is a simple task of two vectors, which has only one solution in vector algebra. Vectors of spin must be positioned along the same line and have opposite directions. If these vectors are not aligned along the same straight line, their sum will not be equal to zero. These two vectors will produce rotational moment. It means that in a Helium atom’s ground state, the vectors of spin for both electrons should be aligned along the line, which connect their positions. For singlet state, the directions of spins are opposite. This statement is strictly correct for para-Helium atoms. For an ortho- Helium configuration, the situation is a little bit more complicated and we shall analyze it below.
Let us take a look below at the directions of electrons spins.
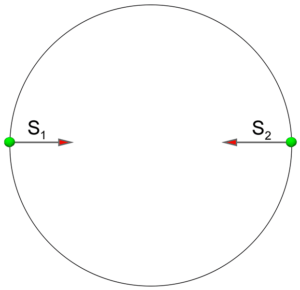
Figure 2a. The sum of vectors of spin aligned along the same line in opposite directions results in a total spin equal to zero in our model.
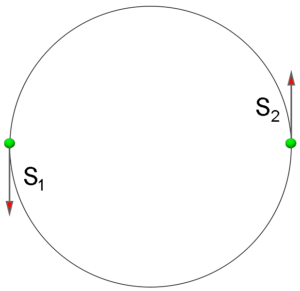
Figure 2b. The sum of up and down vectors of electrons spins is not equal to zero. Combination of these spins results in a new rotational moment in the model, which uses the Pauli Principle.
Vectors of electron spin have a magnetic nature. They behave similar to a compass, which means that they arrange themselves along a stronger magnetic field, created by the orbital movement of electrons. This bring us to conclusion that magnetic vectors of orbital moments in our model should also be directed towards the center of the nucleus.
The continuous travel of an electron along its trajectory induces magnetic fields. In our model, four opposite magnetic fields are created in the length of one round of an electron orbit. These fields are equal in amplitude.
Helium atom.
For the Helium atom we shall use the same model as we used for the Hydrogen atom. The only difference is the double charge of the nucleus and the two of electrons on orbit.
Energy of a moving electron can be expressed from Classic and Quantum Mechanics as:
$\frac {m {\ } v^2}{2} = h \cdot f \cdot n$ (1).
In this formula $m$ – is the electron mass, $v$ – is the electron speed, $h$ – is Planck’s constant, $f$ – is the frequency of an electron wave and $n$ is whole number.
Equation (1) represents the difference between the “rigid rotator” of Quantum Mechanics and our model. We consider each particle with its individual wave, rather than two or three particles with a single combined wave. In our model, waves should interfere between themselves, but cannot be simply added together.
That is why formula (1) is written for each individual electron and it is the same for Hydrogen or Helium atoms.
The length of four hemi spheres must be equal to:
$L = 4 {\ } \pi {\ } r = n \cdot \lambda$ (2).
The frequency of electron orbital rotation can be found as the speed of the electron divided by the length of orbit:
$f = \frac {v}{L} = \frac {v}{4 {\ } \pi {\ }r}$ (3).
Substitution of the frequency from (3) into (1) will result in:
$\frac {m {\ } v^2}{2} = h {\ } \frac {v {\ } n }{4 {\ } \pi {\ } r} = \frac {\hbar {\ } v {\ } n}{2 {\ } r} $ (4a).
We use the reduced Planck constant $\hbar = \frac {h}{2 \pi}$ in expression (4a).
As a result from equation (4) we got the expression for the electron orbital moment:
$m {\ } v {\ } r = \hbar \cdot n$ (4).
Expression (4) means that the electron orbital moment is equal to the whole number, multiplied by the reduced Planck constant. This expression is the same as the one we got for the Hydrogen atom and it means that we do not need the orbital moment postulate for the Helium atom. This conclusion will be important for other atoms of the periodic Table with $s$ type electron orbits in their structure.
In our analysis of the shape of the electron orbit of the Hydrogen atom we came to conclusion that there is no numerical solution for the type of orbit, where the vectors of induced magnetic fields are parallel or perpendicular to $x, y, z$ axes. Such an orbit configuration would contradict the result of equation (4).
Solution for the Faraday equation
$\oint E \cdot ds = – \frac{\partial \Phi _{mag}}{\partial t}$ (5)
we found in the form of the elliptical electron trajectory, projected on the spherical surface.
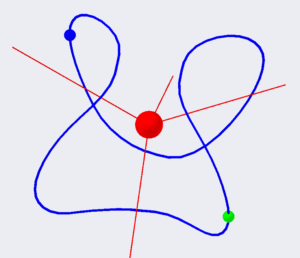
Figure 3. Electrons orbit for the ortho- configuration of the Helium atom.
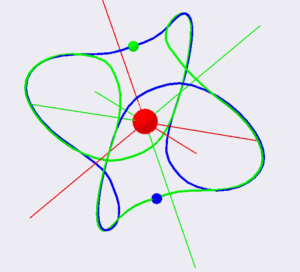
Figure 4. Electrons orbit for the para- configuration of the Helium atom. Green electron moves along the blue line. Blue electron moves along the green line. This was done for better contrast. Straight lines show the direction of induces fields.
In para- configuration, orbits configuration as well as positions of electrons at any moment of time exhibit a point type spherical symmetry. It means that the straight line which connects the positions of electrons, will always cross the center of the nucleus.
The procedure to find the parameters of the electron trajectory is the same as we used for the Hydrogen atom. We have to find the values of three parameters, which define the elliptical trajectory of electrons in the Helium atom and we shall express these values in the units of the radius of the electron orbit.
We start with the ortho- configuration.
Although the values for these parameters, expressed in units of radius are the similar to expressions for the Hydrogen atom, the actual values for the Helium atom are different:
$a = 0.707 \cdot r = \frac {1.414 \cdot \pi \cdot \epsilon_0 \cdot \hbar^2 \cdot n^2}{m \cdot e^2} $, $b = 1.252 \cdot r = \frac {2.504 \cdot \pi \cdot \epsilon_0 \cdot \hbar^2 \cdot n^2}{m \cdot e^2} $ (6).
The energy of the Helium ion, when only one electron is left on the orbit, is the same as was calculated in Bohr’s model:
$E_0 = \frac {m \cdot e^4}{2 \cdot \epsilon_0 \cdot h^2} = 54.4 eV$ (7).
This result is well-known and does not need additional interpretation.
In the case of a Helium atom with two electrons orbiting the nucleus, we start with calculations of the orbit length.
The length of orbit is equal to:
$L = \pi \cdot [3 \cdot (a + b) {\ } – \sqrt {(3a+b) \cdot (a+3b) }] =4 \pi r$ (8).
We used Ramanujan formula for the length of the ellipse in our calculations.
These orbits have three parameters $a, b$ and $r$. Similar to the Hydrogen atom, the values for the parameters $a$ and $b$ can be expressed in the units of the spherical radius $r$ of orbit as:
$a = 0.707 \cdot r$, $b = 1.252 \cdot r$ (9).
The function, which represents the electron trajectory as well as the derivative of this function, are continuous and do not have any singularities.
For two electrons on the surface of the sphere, there is equilibrium between the Coulomb force and centripetal force:
$\frac{2 {\ } e^2}{4{\ } \pi {\ }\epsilon_0 {\ } r^2} = \frac{2 m {\ } v^2}{r} $ (10).
The speed of the electron can be expressed from (10) as:
$v = \sqrt{ \frac {e^2}{4 {\ } \pi {\ } \epsilon_0 {\ } r {\ }m} } $ (11).
Formula (8) guarantees that the expression for orbital moment is correct:
$m \cdot v \cdot r = n \cdot \hbar$ (12).
Combination of (11) and (12) gives us the radius of the spherical surface of the electron orbit:
$m \cdot r {\ } \sqrt{ \frac {e^2}{4 {\ } \pi {\ } \epsilon_0 {\ } r {\ }m} } = n \cdot \hbar$ (13).
$m^2 \cdot r^2 {\ } \frac {e^2}{4 {\ } \pi {\ } \epsilon_0 {\ } r {\ }m} = n^2 \cdot \hbar^2 $ (14).
$m {\ } r {\ } e^2 $=$ 4 {\ } \pi {\ } \epsilon_0 {\ } \hbar^2 {\ } n^2 $ (15).
$r = \frac {4 {\ } \pi {\ } \epsilon_0 {\ } \hbar^2 {\ } n^2}{m {\ } e^2}$ (16).
The energy of two electrons on the Helium orbit can be calculated as:
$E = 2 \cdot \frac {m {\ } v^2}{2}$ (17).
The second power of the electron speed can be expressed from (11) as:
$v^2 = \frac {e^2}{4 {\ } \pi {\ } \epsilon_0 {\ } r {\ } m} = \frac {e^2 \cdot e^2 \cdot m}{4 {\ } \pi {\ } \epsilon_0 {\ } m {\ } 4 {\ } \pi {\ } \epsilon_0 {\ } \hbar^2 {\ } n^2} = \frac {e^4}{4 {\ } {\epsilon_0}^2 {\ } h^2 {\ } n^2} $ (18).
We used expression (16) for the radius of the electron orbit.
From equation (17), the value of the energy of the electron states would be equal to:
$E = \frac {m {\ }e^4}{4 {\ } {\epsilon_0}^2 {\ } h^2 {\ } n^2} = 27.2 eV $ (19).
This value is the energy for the lowest state of the Helium atom in the ortho- configuration. This amount of energy is needed for an electron to reach the ionization level. If we mark ionization energy in the vacuum as zero, then this energy should be negative.
Formula (27) describes the spectrum of the energy levels of the Helium atom in an ortho- configuration. Other energy levels of ortho- Helium for $n > 1$, as well as transitions between them should be observable in Helium spectra, provided excitation method which takes into account spin forbidden transitions between singlet para- Helium ground state and triplet ortho- Helium excited states. Under normal conditions with an optical source of excitation, the spectrum of ortho- Helium lines is practically invisible.
This ortho- state of the Helium atom cannot be the ground state, because both the orbital moment and the spin of the atom in this state are not equal to zero. It means that the Helium atom in this state would be highly reactive and its behavior would be similar to the behavior of a Hydrogen atom.
The ground state of the Helium monoatomic inertial gas belongs to the para-state of Helium.
Para-Helium.
Figure 5 below shows the para- configuration of an electrons orbit of a Helium atom. The orbits of one electron is shown in blue and another in green. These orbits possess a point of symmetry at the center of the nucleus. At any time, two electrons occupy positions on the opposite sides of the diameter of the electrons orbits. The directions of orbital moments, as well as the directions of induced magnetic fields are indicated by four red lines for one electron and by four green lines for another electron. The angle between any two lines of the same color is approximately 109.47 degrees. The directions of two momentums and two magnetic fields for each electron are to the center of the sphere and two other vectors have directions away from the spheres center.

Figure 5. Electrons orbits of a Helium atom in the para- configuration.
Figure 5 shows the orbits of electrons in the para-configuration of a Helium atom. Green and blue electrons are located at opposite sides of the diameter of their orbit. Their orbits are symmetric relative to the proton position. The directions of the induced magnetic field are shown as the green and blue lines.
For the para- configuration of an electron orbits total spin, orbital momentums as well as the integrals of electric and the induced magnetic field are equal to zero.
As a result, a Helium atom in the para- configuration of orbit occupies a stable energy state and no external interaction is needed in order to compensate the non balanced orbital moments and spin fields. That is the reason for Helium atoms in the para-configuration being noble, inertial & monoatomic gas.
In order to find the value of the energy in the para- configuration, we need to multiply the value of an electrons energy in an ortho- configuration by the steric vector coefficient (see next paragraph):
$k = \frac {1}{2} \cdot (1+ \frac {\sqrt 2}{\sqrt 3}) = 0.909 $ (20).
The energy of the ground state of a Helium atom is equal to the energy of the lowest state in the para- configuration:
$E_0 = 27.2 \cdot \frac {1}{2} \cdot (1+ \frac {\sqrt 2}{\sqrt 3}) = 24.7 eV$ (21).
This result agrees with the experimental value for ionization energy of the first electron of a Helium atom, which is equal to $E_{ionization} = 24.6 eV$. The difference of energies of about $\Delta E = 0.1 eV$ should be attributed to spin-spin interaction.
Although in the ground state, a Helium atom exists only in the para- configuration. The excited states of both configurations can be observed in spectral data, although transitions between these two states cannot be observed in the case of optical excitation because they are spin forbidden. Electron excitation would solve that problem and it would be possible to observe levels for both states.
Calculations of steric vector coefficient for para- Helium.
In our calculations of the electron orbital moment, we used the principle of independence of orthogonal components of an electrons movement. Without a special statement, we assumed that components which are orthogonal to the orbital component, give no input into total energy of electrons in a Helium atom. We calculated the energy of two electron systems as if total energy is combined as a scalar function of orbit radius and neglected the vector character of angular components of an electrons trajectory.
From a Classic Mechanics point of view, such an approach looks justified, because two electrons in a Helium atom are positioned at the opposite ends of the diameter of their orbits. The same argument could be said for Quantum Mechanics, which represents electrons as a distributed cloud, where positions of every electron cannot be defined or determined.
But our calculations are based on Electrodynamics.
The energy of an electron in an electric field can be calculated as field potential multiplied by the charge of the electron:
$Energy = E \cdot e$ (22).
This expression describes the potential energy. It will become the energy of the electron after the electron passes the distance along the field with such potential.
According to the Faraday formula, the magnetic field induced by moving charges is equal to:
$\oint E \cdot ds = – \frac{\partial \Phi _{mag}}{\partial t}$ (23).
This Faraday formula gives us the chance to produce the rules of addition of vector expressions, which are proportional to the energies of each electron. Instead of a three dimensional integral of an electric field, we shall find the sum of the vectors of induced magnetic fields for the first electron and the vector of induced magnetic field for the second electron because these values are in direct proportion. Then we shall use the steric coefficient which we find for induced magnetic vectors in order to combine the energy of electrons.
The value of energy for each electron in equation (24) is equal to one half of the total energy, which we found for the ortho- configuration of the Helium atom:
$E_1 = E_2 = \frac {27.2}{2} $ (24).
The energy of two electron systems will be equal to the energy of the first electron plus the energy of the second electron, multiplied by the steric vector coefficient:
$Energy = E_1 +k \cdot E_2 = \frac {1}{2} \cdot (E_1+k \cdot E_2)$ (24).
Induced magnetic fields for each electron in a Helium atom have the geometry of the cube with the angles of 109.47 degrees between the directions of induced magnetic fields. It means that each vector of induced magnetic field can be represented by a line from the center of the cube to a non-adjacent corner of the cube:
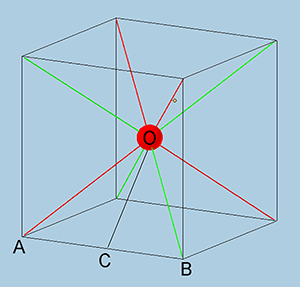
Figure 6. illustrates the case for two electrons with their orbits having point symmetry at the center of the cube.
The red sphere represents the Helium nucleus. The red lines show the direction of the induced magnetic field for one electron. The green lines show the direction of the induced magnetic fields for the other electron. Out of the four vectors for each electron, two vectors have direction towards the nucleus and the two other vectors have direction towards the corner of the cube.
The steric coefficient can be calculated from Figure 6. If we assume that the length of the side of the cube as 2a unit, then the length of the diagonals AO and BO would be equal to:
$AO = BO = a \cdot \sqrt 3$ (26).
Half sum of these two momentums or the line OC has the length:
$OC= \frac {1}{2} (AO + BO) = a \cdot \sqrt 2$ (27).
It means that in order to add the vector momentum of a second electron to the vector of the first electron it is necessary to multiply the vector of the second electron by the steric coefficient:
$k = \frac {1}{2} \cdot (1+\frac {\sqrt 2}{\sqrt 3}) = 0.908 $ (24).
$E_{para} = \frac {1}{2} \cdot (E_1 + E_2 \cdot \frac {\sqrt 2}{\sqrt 3}) = 24.7 eV$ (27).
*************************