Research
Our group is currently engaged in three projects: (1) Inhibition of protein-protein interactions for the discovery new classes of therapeutics, (2) Development of an encodable scaffold for duplex RNA recognition, and (3) Design of organocatalysts for peptide and protein synthesis.
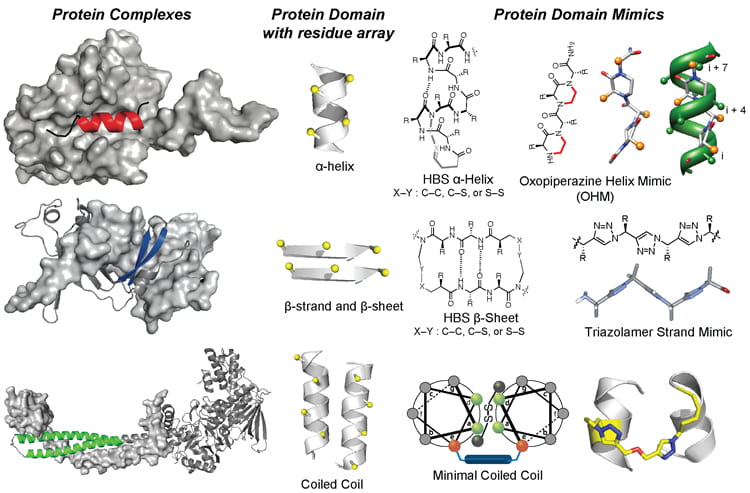
A major focus of our group is to develop a systematic approach for targeting protein-protein interactions with synthetic ligands. Protein-protein interactions are considered challenging targets because of the inherent difficulty in specifically recognizing large interfaces with small molecules. We have developed three classes of synthetic scaffolds that mimic helical, strand, sheet and coiled-coil conformations. The synthesis of these scaffolds utilizes alpha amino acids and has been optimized for solid phase. Several examples in which the designed molecules can modulate intracellular protein-protein interactions have been described. We have termed these compounds, Protein Domain Mimics (PDMs). As part of our overall efforts, we have also computationally identified protein-protein complexes that are potentially amenable to disruption by synthetic mimics of protein secondary structures. For a recent review of our efforts on the development of PDMs, please see: Acc. Chem. Res. 2017, 50, 1313-1322.[PDF]
The image below shows native protein complexes, the domains that may be mimicked for inhibition of the target complex and our designed PDMs. The design, synthesis, solution conformations and the biological potential of each scaffold is discussed in our publications.